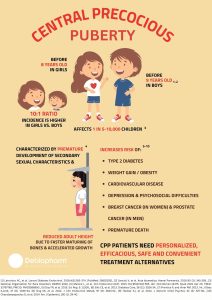
Central Precocious Puberty
Central precocious puberty is a condition that causes early sexual development in girls and boys. Puberty normally starts between ages 8 and 13 in girls and 9 and 14 in boys. In girls with central precocious puberty, signs of pubertal development are visible before age 8 and in boys with this disorder they begin before age 9.
Signs of puberty include development of pubic and underarm hair, a rapid increase in height (commonly referred to as a “growth spurt”), acne and underarm odor. Girls also develop breasts and begin their menstrual periods. Boys have growth of the penis and testes and deepening of the voice.
Because of the early growth spurt, children with central precocious puberty may be taller than their peers; however, they may stop growing abnormally early. Without proper treatment, some affected individuals are shorter in adulthood compared with other members of their family. Developing ahead of their peers can be emotionally difficult for affected individuals and may lead to psychological and behavioral problems.
Clinical trials
A ‘clinical trial’ is a research study in which people agree to test a potential new treatment to prevent or improve a disease or medical condition. A clinical trial also looks at how participants react to the potential new treatment and if any unwanted effects occur. This helps to determine if the new investigational treatment works, is safe, and is better than those that are already available. Many clinical trials also compare existing treatments or test new ways to use or combine existing treatments.
All new drugs must be tested in clinical trials before they can be prescribed to patients. Without children and people taking part in these research studies, we would have no new drugs to help others with their condition. Diverse groups of participants are included in clinical trials because drugs may affect people differently based on their age, sex, gender and ethnicity.
The Libelula TM clinical trial of Debio 4326 for children with central precocious puberty (CPP)
The LibelulaTM clinical trial will assess a new drug (Debio 4326) for children with central precocious puberty (CPP). Girls and boys with CPP may have social and emotional problems because they feel different from their peers. Hormonal treatment to slow down puberty can last for several years, and the existing injectable treatments need to be taken every 1, 3, or 6 months. In this clinical trial, the study drug will be given once a year.
Your child may be able to take part if they:
- are aged 5 to 8 years, inclusive
- have CPP, and
- are currently on a gonadotropin-releasing hormone agonist (GnRHa) therapy or are untreated (treatment-naïve) for CPP.
Children who can join the clinical trial might be receiving treatment for CPP, or they might not yet be getting any hormonal treatment.
Children of other ages may later (after an interim analysis) be able to take part in this clinical trial. When contacting a clinical trial doctor, they will be able to tell you if children under 5 or older than 8 can now take part in the study.
Participants will receive 2 injections of Debio 4326 within the planned 2-year study period followed by a 12-week post-treatment period. Participants will have a maximum of 21 study visits.
Information on Triptorelin history
The LibelulaTM study drug (Debio 4326) is triptorelin, which belongs to the gonadotropin-releasing hormone agonist (GnRHa) family used as standard of care in Central Precocious Puberty. GnRHa treatment can reduce the level of sex hormones and slow down the progression of puberty and physical development.
The LibelulaTM study drug formulation is designed as an extended-release formulation of triptorelin (12-month formulation) and will be administered as an intramuscular injection. An extended-release formulation means that the drug is slowly released in the body over a prolonged period of time.
With this new formulation, the LibelulaTM Study intends to further reduce the frequency of injections, and consequently the frequency of potential injection-site reactions. Moreover, this formulation will be especially beneficial to children who are diagnosed with CPP very early on in their childhood and require long-term GnRHa treatment to suppress the progression of the precocious puberty.
-
Phases
When a potential new medication is being developed, it is first tested in a laboratory setting. If the results are positive, the drug may enter a clinical trial program. This means that it will be tested in humans in several ‘phases’ of study.
Phase I = Safety evaluation. The very first administration in humans, typically carried out in a small group of healthy volunteers to assess if the drug is safe.
Phase II = Efficacy evaluation. The first trials in patients with the intended disease to check if the drug works efficiently and if there are any unwanted side effects.
Phase III = Confirming findings. Trials in large numbers of patients that generally compare the drug to the best treatments available.
_Phases
- P
- Ⅰ
- Ⅱ
- Ⅲ
- M
Talk with your medical doctor
If you are interested in participating in an upcoming clinical trial (can be also referred to as a clinical study), ask your doctor if a clinical trial might be right for you. Your doctor knows both you and your health history, which is invaluable in making this decision. Your doctor can help you gather the information needed to locate a trial and help you identify what questions might be important to ask the clinical trial doctor before deciding to participate.
Find the trials on the map
This map relies on Google Maps, which could install cookies.
Please accept them in order to show the map.
Clinical trial of triptorelin in children with precocious puberty
Debiopharm International has conducted a Phase III trial of triptorelin Definition Triptorelin, the active substance of Decapeptyl®/Trelstar®/Pamorelin®, is a GnRH agonist analogue. An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. More info: http://bit.ly/2vO2Px7 22.5 mg 6-month formulation in children with precocious puberty, a hormonal Definition a hormone is a substance produced by a gland that regulates other parts of the body disorder causing early pubertal changes Definition development of pubic hair and breasts and onset of menstrual periods in girls, and increased growth of testicles and penis in boys and accelerates growth speed and maturation of the bones. The purpose of this trial was to show that triptorelin Definition Triptorelin, the active substance of Decapeptyl®/Trelstar®/Pamorelin®, is a GnRH agonist analogue. An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. More info: http://bit.ly/2vO2Px7 works and is safe in children with precocious puberty when it is injected by the intramuscular route (i.e. in the muscle) every six months (triptorelin Definition Triptorelin, the active substance of Decapeptyl®/Trelstar®/Pamorelin®, is a GnRH agonist analogue. An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. More info: http://bit.ly/2vO2Px7 6-month formulation). The treatment in this study lasted 1 year, each child being injected twice with the triptorelin Definition Triptorelin, the active substance of Decapeptyl®/Trelstar®/Pamorelin®, is a GnRH agonist analogue. An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. More info: http://bit.ly/2vO2Px7 6-month formulation. This indication has since been registered in Europe and the submission dossier registration is currently being examined in the US.
Triptorelin has been used for the treatment of precocious puberty since 1986, especially in Europe. The effect of triptorelin Definition Triptorelin, the active substance of Decapeptyl®/Trelstar®/Pamorelin®, is a GnRH agonist analogue. An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. More info: http://bit.ly/2vO2Px7 is to reduce the quantity of the hormones in the body responsible for the early pubertal changes described above. In the beginning, triptorelin Definition Triptorelin, the active substance of Decapeptyl®/Trelstar®/Pamorelin®, is a GnRH agonist analogue. An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. More info: http://bit.ly/2vO2Px7 was given as injections administered every month or every three months. The triptorelin Definition Triptorelin, the active substance of Decapeptyl®/Trelstar®/Pamorelin®, is a GnRH agonist analogue. An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. More info: http://bit.ly/2vO2Px7 preparation that was administered in this recent trial is similar to the triptorelin Definition Triptorelin, the active substance of Decapeptyl®/Trelstar®/Pamorelin®, is a GnRH agonist analogue. An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. More info: http://bit.ly/2vO2Px7 injected every month or every three months except that it can be injected every six months. These preparations are all sustained-release formulations produced in such a way that once the drug has been injected, the drug is released in the body continuously over the indicated period.
-
Phases
When a potential new medication is being developed, it is first tested in a laboratory setting. If the results are positive, the drug may enter a clinical trial program. This means that it will be tested in humans in several ‘phases’ of study.
Phase I = Safety evaluation. The very first administration in humans, typically carried out in a small group of healthy volunteers to assess if the drug is safe.
Phase II = Efficacy evaluation. The first trials in patients with the intended disease to check if the drug works efficiently and if there are any unwanted side effects.
Phase III = Confirming findings. Trials in large numbers of patients that generally compare the drug to the best treatments available._Phases
- P
- Ⅰ
- Ⅱ
- Ⅲ
- M
Please share any related associations that may be useful